|
|
|
Copyright ©1998 by The Resilience Alliance*
Nocera, J.J. and P.D. Taylor. 1998. In situ behavioral response of common loons associated with elevated mercury (Hg) exposure. Conservation Ecology [online] 2(2): 10. Available from the Internet. URL: http://www.consecol.org/vol2/iss2/art10/
A version of this article in which text, figures, tables, and appendices are separate files may be found by following this link.
Research In Situ Behavioral Response of Common Loons Associated with Elevated Mercury (Hg) Exposure Joseph J. Nocera and Philip D. Taylor
Acadia University
- Abstract
- Introduction
- Methods
- Results
- Discussion
- Speculation
- Responses to this Article
- Acknowledgments
- Literature Cited
Common Loons (Gavia immer) in Nova Scotia, Canada have the highest blood mercury (Hg) concentrations of any loon population in North America. Previous studies have shown that exposure to varying levels of Hg in prey is associated with changes in pre-nesting adult behavior. We report here the first association of sublethal blood Hg contamination with changes in behavior of Common Loon young. As Hg levels in their blood rise, the amount of time that chicks spend brooding (by back-riding) decreases (P = 0.004) and time spent preening increases (P = 0.003). The sum increase in energy expenditure is not being compensated for with expected increases in feeding rates or begging. We suggest that such altered time-activity budgets may disrupt the energetic balance of young. Our results show that variation in time spent back-riding is associated with changes in fledging rates. Adult behavior did not significantly vary with Hg, but results are suggestive that an association may exist. We also show that monitoring the time-activity budgets of very young chicks can serve to indicate the effects Hg concentrations in their blood. We confirm the hypothesis that loons and other upper trophic level predators could be at risk from elevated levels of bioavailable Hg. This may help to explain the chronically low productivity of such contaminated sites as Kejimkujik and allow for more focused management initiatives.
KEY WORDS: behavioral toxicology; common loon behavior; Gavia immer; Kejimkujik National Park; Nova Scotia; Canada; mercury; neurotoxicity; southwestern New Brunswick; sub-lethal exposure; time-activity budgets.
Mercury is a volatile toxicant because of its persistence and high mobility in the environment. It can be transmitted over long ranges in the atmosphere and deposited far from its source. It can be biologically transformed into methylmercury (a highly toxic compound) and bioaccumulates in top predators, including humans. Human activities that release mercury into the environment therefore can pose significant dangers to both human health and wildlife.
A large contribution of environmental mercury is made through anthropogenic deposition as a by-product of fossil fuel combustion, municipal waste incineration, and other industrial processes (Swain et al. 1992, Carpi 1997). Ubiquitous contaminants that artificially enter ecosystems put all wildlife at risk, particularly those that are upper trophic level predators, such as loons. Monitoring the behavior of such animals can provide an indication of the sublethal effects of toxic substances on an organism, and is a practical means of assessing the ecological impact of certain toxicants (Døving 1991, Cohn and MacPhail 1996).
Blood mercury (Hg) levels of the Common Loon (Gavia immer) in the northeastern United States and Atlantic Canada, particularly Kejimkujik National Park (Nova Scotia, Canada), are the highest known among tested loon populations in North America (Burger 1994, Beauchamp et al. 1997, Evers et al. 1998). Kejimkujik has one of the most easterly North American loon populations, and blood Hg levels have shown a trend of easterly increase (Evers et al. 1998). Increases in atmospheric deposition of mercury during the past century have been documented (Swain et al. 1992) and it is known that mobilization of mercury currently exceeds that which occurs naturally (U.S. EPA 1997, Vitousek et al. 1997).
Loon reproductive success at Kejimkujik (Kerekes 1994; N. Burgess, unpublished data) is low compared with that of other North American populations, most notably among those in Eastern Canada (Scheuhammer et al. 1998). Mercury is a known neurotoxin that has been observed to affect avian behavior and cause aberrant breeding responses (Heinz 1976, 1979) when consumed in prey or forage. It may result in reduced productivity through lack of a nesting attempt (Barr 1986, Scheuhammer and Blancher 1994), lower nest attentiveness (Barr 1986), or lower nest site fidelity (Tejning 1967). However, no previous studies have assessed the effects of Hg contamination on the in situ behavior of loons or on potential population effects of any behavioral changes. Such information is relevant to the management of viable populations by identifying and indicating potential impacts of contaminants, such as mercury exposure.
Because Common Loons are long-lived piscivores and are a K- selected species, impacts of low productivity on a population may not be seen for many years. Given its low productivity (Kerekes 1994; N. Burgess, unpublished data), Kejimkujik may act as a sink for Common Loon reproduction even though requisite nesting habitat is found in abundance, namely, large (>40 ha) fish-containing, oligotrophic lakes with island nesting areas that are relatively free from predators and human disturbance (McIntyre 1983, Belant and Anderson 1991, Kerekes 1994). The implications of a sink population in Kejimkujik may be that this site drains regional populations of potentially successful breeding loons, adding yet another stress point to a species whose range is progressively decreasing (Sutcliffe 1978, Blair 1992). Reasons for low reproductive success at this site have yet to be identified.
In contrast, loon populations in southwestern New Brunswick, Canada have higher productivity and much lower blood Hg levels than in Kejimkujik (Beauchamp et al. 1997). A strong correlation of blood Hg within families suggests that it is being accumulated on these summer territories (Beauchamp et al. 1997). Therefore, Hg should exert the strongest effects during the breeding season, with potential negative consequences for overall productivity. We tested the hypothesis that any such effects might reveal themselves through subtle, altered behaviors of adults or chicks. We address this question through a comparative, cross-generational investigation of breeding, nonbreeding, and failed-breeding loon behavior across a range of lakes that exhibit variation in lake morphometry, lake chemistry, and biological characteristics in Kejimkujik and the Lepreau Crown Lands (southwestern New Brunswick).
We collected time-activity budgets (TABs) (Altmann 1974, Tacha et al. 1985) and event behavior (Martin and Bateson 1993) quantifications to catalog pre-/post-hatch and pre-/post-nesting behavioral states (Martin and Bateson 1993) of 12 breeding pairs (24 adults and 16 chicks) of Common Loons in Kejimkujik National Park, Nova Scotia, Canada (44°20' N, 65° 20' W), and the Lepreau watershed, New Brunswick, Canada (45°20' N, 66 °35' W), that resided on 11 territories of varying chemistry, morphometry, and Hg levels in yellow perch and loon blood (one breeding pair was sampled in both years). We collected the same data for 14 pairs of nonbreeding and four pairs of failed-breeding loons that resided on 15 lakes at the same sites.
We observed Common Loon behavior on both study areas for 264 h (n = 295 observations, mean observation time 53.7 min per bout) from May to September of 1996 and 1997. Behavioral observations were made with 8x42 binoculars or a 20-60x spotting scope from a concealed location (Bradley 1985) from shore (with a good view of the nest, brood, resident pair, or individual). Diurnal observation times were divided into 3-h maximum slots ranging from 0600-0900 to 1800-2100 AST (Atlantic Standard Time) in 1996 and 1-h maximum slots ranging from 0600-0700 to 2000-2100 AST in 1997. At least two behavioral observations were done per day, allowing for every lake to be covered at least once every 9 d. All individuals were randomly chosen for each observation and concurrent observations were made when individuals could be positively identified. Observations conducted during the development stages of chicks were divided among three age classes: downy young, DY (<13 d old); small young, SY (13-40 d old); and large young, LY (>40 d old).
Previously described loon behaviors (Sjølander and Ågren 1972, McIntyre 1975, Evers 1994) were documented by dictating observations into a microcassette recorder, along with times recorded using a 1/100 s stopwatch. Behaviors that lasted >5 s were deemed states; behaviors that lasted <5 s were considered events. We quantified the following states: swimming (all active and passive locomotory behaviors, e.g., swimming, drifting, and flying), incubation, preening, diving, brooding, territorial encountering, and bathing (both were combined to form the category "other"). We quantified the following behavioral events: foot waggle, stretching, vulture posturing, splash diving, peering, yawning, vocalizing (wails, tremolos, hoots, yodels, and mews), penguin dancing, and begging.
Our observed behavioral states did not include time spent in observation with the birds out of sight. "Out of sight" is a category that has sometimes been included in TABs (e.g., Evers 1994), but it was not included in this study because we felt that the information gained from quantifying "out-of-sight" times would be minimal, if not detrimental, by biasing toward a category that is not a behavior, but a ramification of observer location. There was no reason to believe that particular discrete behaviors were occurring during "out of sight" times, which is an acknowledged danger (Martin and Bateson 1993) regarding secretive behaviors of select animals (such as cats, Felis domestica, procuring prey and taking it elsewhere to eat). Therefore, we removed out-of-sight times and reduced the sampling period accordingly (as did Gese et al. 1996).
To help avoid the potential problem of individual observer bias and misinterpretation of behaviors (Martin and Bateson 1993), we trained all field assistants for 5-7 d (watching a loon simultaneously to define behaviors) to establish a consistent protocol before they proceeded to collect data independently (as did Gese et al. 1996). Because Common Loons are not sexually dimorphic and many of our study subjects were unbanded, we were unable to investigate gender differences in behavior. However, Mager (1995) demonstrated that male and female loons exhibit minimal difference in behavior.
We chose to use continuous observations and focal animal sampling (Altmann 1974, Martin and Bateson 1993) because they are the only reliable methods of calculating actual activity patterns (Winchell and Kunz 1993). These measures offer a more complete and descriptive data set than do instantaneous and/or nonfocal animal sampling (Martin and Bateson 1993). Behaviors that are infrequent can often be missed during scan and instantaneous sampling and can only be accurately quantified with focal continuous observation (Winchell and Kunz 1993).
Independent variable compilationWe first categorized lakes using Principal Components Analyses (MathSoft 1995). These were conducted for both the morphometric and chemical variables (Table 1) to limit the analysis against behavior to a smaller number of linear combinations of those variables. All principal components with composite eigenvalues below the eigen mean (= 1) were excluded from the analyses (MathSoft 1995). Chick blood was sampled and Hg concentrations were determined (N. Burgess, unpublished data) for six of our 11 territories sampled for behavior; adult blood was sampled for seven of 11 territories. On one of our territories, no chick or adult blood was sampled, so this territory was eliminated from all further analysis. Hg concentrations in chick blood for the missing four of the remaining 10 territories was estimated by fitting a linear regression of Hg in chick blood on Hg in adult blood, using an extended data set with measurements from 19 adults with 14 chicks in 13 territories on lakes throughout the two study areas (N. Burgess, unpublished data). The three missing samples of Hg in adult blood were likewise predicted. Such an approach is justified; theoretically, chicks have Hg levels that are related to those of their parents, because adults depurate Hg to the embryo (Heinz 1987). Empirically, the relationship between Hg in adults and chicks bears this out.
|
Table 1. List of independent variables used to test for variation with loon behavior on respective lakes. Data were collected by the Canadian Wildlife Service (N. Burgess, unpublished data), Parks Canada (Beauchamp et al. 1997), or were extracted from Kerekes and Schwinghammer (1973) and from McNicol et al. (1996).
|
All blood samples were also screened for organochlorines, lead, and polychlorinated biphenyls (N. Burgess, unpublished data). These samples showed normal levels of these contaminants, with only Hg being irregularly high. Therefore, we only used Hg in our analyses. Observations of the presence of predators, humans, inter-, and intraspecifics were also quantified for each lake for an individual observed on a unit time (100-min) basis.
Statistical analysisAll percentage data from the TABs were arcsine square-root transformed. Then we tested for effects of the predictors (independent variables) on the response (loon behavior as TABs and events), using MANOVA (MathSoft 1995) to elucidate any potential effects. We chose Pillai's criterion (Hair et al. 1987), which considers all characteristic roots, for significance testing in MANOVA. To further elucidate which (if any) discrete behavioral state(s) or event(s) were affected by these predictors, we performed multiple univariate ANOVAs as the final step. For these analyses, significant correlation probability values were assumed at a level of confidence less than or equal to 0.01 (Bonferroni correction; Sokal and Rohlf 1995).
To examine the influence of behavioral patterns on reproductive success, we used any significantly modified behaviors as predictive variables in a linear regression with reproductive success estimates.
The first PCA reduced the 15 lake morphometry variables (Table 1) to three components that explained 89.7% of the original variation in those variables. The first morphometrical principal component (MPC1) was loaded heavily by all morphometric variables; the second (MPC2) was loaded heavily by volumetric parameters such as flushing (flow rate) and basin area; and the third (MPC3) was loaded primarily by variables describing shoreline length and complexity and area.
A second PCA reduced 12 lake chemistry variables (Table 1) to three components that explained 76.7% of the original variation in those variables. The first chemical principal component (CPC1) was loaded heavily by pH, SO42-, and chlorides; the second (CPC2) was heavily loaded by all chemical variables, but was influenced most by conductivity, phosphorus, and calcium. The third component (CPC3) was loaded primarily by variables describing water clarity (e.g., color and total organic carbon) and nitrogen.
Using MANOVA, we then separately tested how each of the three chemical and three lake morphometry principle components and seven other biological variables influenced TABs and event behaviors of adult loons. These analyses revealed that event behaviors of pre-nesting, non-incubating and post-hatch adults were influenced by the presence of interspecifics in territories (Pillai trace = 0.979, P = 0.042). Secondly, TABs of breeding adults seemed to be marginally associated with blood Hg (Pillai trace = 0.847, P = 0.087). The TABs of nonbreeding adults were not significantly associated with any predictor. However, event behaviors were significantly related to predator threat and intraspecific competition (Pillai trace = 0.989, P = 0.04 and Pillai trace = 0.999, P = 0.002, respectively).
Further multiple univariate ANOVA showed that the only event behavior of breeding adults that was modified by interspecific presence was the hooting vocalization (P = 0.03). Predators were correlated with the occurrence of nonbreeder stretching events (P = 0.02). The relationship of stretching events with predator presence was driven by two outliers (the only two nonzero points). When these outliers were removed from the analysis, the significant relationship was lost (P = 0.4). Lastly, intraspecific competition was correlated significantly with yodeling events (P less than or equal to 0.01). Again, the significant effect was lost when a single outlier (the only nonzero point) was removed from the analysis (P = 0.5).
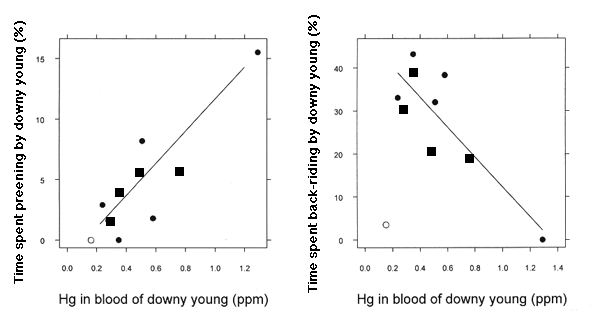
A second set of analyses on TABs of chicks at all developmental stages (Table 2) revealed a relationship between Hg burdens in chick blood (0.15 - 1.29 ppm) and TABs patterns of downy young <12 d old (P = 0.044). Additional analyses (Table 3) show that this was manifest as a significant negative effect (P = 0.004) of chick blood Hg burden on time spent by chicks in back-riding, i.e., brooding by riding on a parent's back (McIntyre 1975), and a positive effect on time spent preening (P = 0.003; Fig. 1). One observation had very high influence and leverage; it was removed from this analysis (Table 3) and from all further discussion. The chicks in this territory had very low mercury burdens, yet spent little time back-riding (Fig. 1). This territory had no secluded nursery areas, and three large boat launches were present, creating an inordinate amount of human disturbance. A second observation, with the lowest estimate of back-riding and highest estimate of preening, had moderate leverage and influence, but was not removed from the analysis; excluding this point did not change the magnitude and direction of the regression coefficients (Fig. 1). The point's leverage is due to a lack of behavioral data from chicks with similarly high blood Hg.
|
Table 2. Results of MANOVA on time-activity budgets of downy young (>12-d-old) Common Loon chicks. Predictive variables were compiled separately for each lake. CPC1-3 are the first three principal components of lake chemistry. Time-activity budgets quantify the proportion of time spent in various behavioral states. Pillai's Trace criterion for significance testing in MANOVA was used. Maximum probability (Type I) error was set at 0.05.
|
|
Table 3. Multiple univariate ANOVA exploration of significant interaction between time-activity budgets of downy young chicks and Hg burdens in their blood (pre- and post-removal of a single outlier with high influence and leverage). Significant P values are indicated in boldface.
| ||||||||||||||||||||||||||||||
We then tested for any relationship between modified behaviors and reproductive success estimates (Kerekes 1994, N. Burgess, unpublished data) from the same set of lakes. A marginal positive relationship (P = 0.024) of back-riding with the ratio of fledged chicks per hatched nest was revealed (F = 3.79, P = 0.087). Fledging rates (range: 0 - 1.67 fledged chicks/hatched nest) differed by about one chick per nest over the range of back-riding time observed (0% to 43%).
Previous laboratory studies have shown that parent/offspring interactive behaviors are modified by in vivo mercury levels through decreases in responsiveness to parental vocalizations (Heinz 1979). Our results, which show that back-riding time decreases as blood Hg increases, add to this argument: we believe that back-riding is a similar composite interactive behavior. Increases in preening may function either as a random displacement activity or may result from increased exposure to wave action as time spent back-riding declines. However, a lack of correlation between back-riding and preening times suggests that random displacement is more plausible.
We were unable to indubitably specify that Hg affects only chick behavior as the relationship between adult TABs and blood Hg approached significance. Future investigations might move toward developing a more sensitive assay for these behaviors to describe whether chicks are soliciting rides less, or whether the instances of adults offering rides decreases as Hg increases.
Nevertheless, we suggest that these results support the hypothesis that loons are affected by exposure to elevated levels of bioavailable Hg (Meyer et al. 1995) through behavioral modifications. Brooding young by back-riding protects chicks from underwater predators, facilitates thermoregulation, and serves as an energy-saving strategy (McIntyre 1975). Any reduction in the frequency of this behavior exposes young to increased predatory threat and increased energy expenditure. Increases in preening time also draw from the energy supply of young. Concomitant increases in feeding or begging rates that would compensate for any increased energy expenditure were not observed. The marginal relationship between modified chick behaviors and reproductive success estimates adds further evidence that decreases in back-riding times may be affecting chick survival by any combination of these factors. We propose that this relationship between back-riding times and fledging rate indicates a biologically significant effect that warrants further study.
Altered behaviors of chicks may serve as an indicator of sublethal Hg exposure on natal lakes, and we now have indirect evidence that they serve as an indicator of survivorship. In addition, Barr (1986) found that Hg exposure from prey has a negative relationship with the occurrence of nesting. Without nesting, no chicks will be produced to serve as sublethal Hg exposure indicators. There is probably a critical threshold of Hg burden and altered loon behaviors (which serve as an indicator of sublethal exposure) and a higher threshold at which there is zero productivity. Loon chicks in this study with the highest Hg exposure (1.29 ppm in blood) spent no time being brooded by parents (Fig. 1). We suggest that exposure of this magnitude (1.25 - 1.50 ppm) is at, or near, a critical behavioral and/or lethal effect level for chicks.
Monitoring the behavior of animals can provide a sensitive indicator of the effects of toxins on an organism (Pfister et al. 1992). We propose that the kind of quantitative, in situ observational methodology that we used could be used in other taxa as indicators of environmental stress by toxicants. Such behavioral studies do not preempt physiological investigations of organismal toxicity. Use of both methods in conjunction in laboratory or field studies can provide a means to accurately portray the full influence of animal intoxication (Cohn and MacPhail 1996).
We did not detect a response to Hg by adults, but the marginal relationship that was revealed may indicate an association. Perhaps chicks may be back-riding less due to fewer solicitations, or parents may be decreasing the rate at which they offer rides, or perhaps it is a combination. However, we are certain that back-riding is affected and that chicks preen more often as Hg increases. How Hg elicits these responses is unknown to us. One plausible pathway is through endocrine dysfunction, which has been associated with Hg contamination in wildlife (Facemire et al. 1995, Hontela et al. 1995), especially in developing organisms, and with subsequent lack of reproductive success. Future research should examine whether endocrine dysfunction in loons with high Hg burdens is an issue, and whether subsequent behavioral modifications of adults and/or chicks are primary or secondary to that dysfunction.
Recently described anthropogenic increases in environmental mercury contamination (U.S. EPA 1997) are probably associated with the high levels of Hg in the blood of loons in Kejimkujik and elsewhere,such contamination surely extends to other top-end predators. Our demonstration of sublethal Hg-associated behavioral changes does not implicate any particular source of environmental Hg. However, we suggest that further control of mercury emissions from industrial sources would be a move toward prevention of further environmental degradation and adverse effects on wildlife. This research should provide an impetus for future investigation of in situ animal behavioral toxicology and the identification of the major Hg pollution sources in North America.
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a comment, follow this link. To read comments already accepted, follow this link.
Acknowledgments
We thank those who assisted in data collection: M. Duggan, J. Scott, M. Tilley, N. Benjamin, and C. Frail (who also spent many patient days helping with data entry). This study was supported by the Canadian Wildlife Service - Atlantic Region (N. Burgess, project coordinator) and the Science Horizons Program of Environment Canada. Additional funding was received from the Explorers Club, Wilson Ornithological Society, and the Association of Field Ornithologists. Our gratitude also goes to Parks Canada and the Kejimkujik National Park staff for assistance with logistics and supplying perch Hg data. N. Burgess (who graciously provided most predictive variable data) and J. Paruk gave helpful comment on early drafts of this manuscript. I. Jonsen and M. Yorke aided with graphics creation. We are grateful for the comments of three anonymous reviewers that enhanced the final manuscript.
Altmann, J. 1974. Observational study of behavior: sampling methods.Behaviour 49:227-267.
Barr, J. F. 1986. Population dynamics of the Common Loon (Gavia immer) associated with mercury contaminated waters in northwestern Ontario. Occasional Paper Number 56, Canadian Wildlife Service, Ottawa, Ontario, Canada.
Beauchamp, S., N. Burgess, A. D'Entremont, R. Tordon, G. Brun, D. Leger, W. Schroeder, and J. Abraham. 1997. Mercury in air, water and biota in Kejimkujik National Park, Nova Scotia, Canada. Proceedings of the Third International Conference of Science and the Management of Protected Areas, 12-16 May 1997. Calgary, Alberta, Canada.
Belant, J. L., and R. K. Anderson. 1991. Common Loon,Gavia immer, brood habitat use in northern Wisconsin. Canadian Field-Naturalist 105:372-375.
Blair, R. B. 1992. Lake features, water quality and the summer distribution of Common Loons in New Hampshire. Journal of Field Ornithology 63:1-9.
Bradley, D. W. 1985. The effects of visibility bias on time-activity budget estimate of niche breadth and overlap. Auk 102:493-499.
Burger, J. 1994. Heavy metal concentrations in feathers of Common Loons (Gavia immer) in the northeastern United States and age differences in mercury levels. Environmental Monitoring and Assessment 30:1-7.
Carpi, A. 1997. Mercury from combustion sources: a review of the chemical species emitted and their transport in the atmosphere. Water, Air, and Soil Pollution 98:241-254.
Cohn, J., and R. C. MacPhail. 1996. Ethological and experimental approaches to behavior analysis: implications for ecotoxicology. Environmental Health Perspectives 104 (Supplement 2):299-305.
Døving, K. B. 1991. Assessment of animal behaviour as a method to indicate environmental toxicity. Comparative Biochemistry and Physiology 100 C(1/2):247-252.
Evers, D. C. 1994. Activity budgets of a marked common loon (Gavia immer) nesting population. Hydrobiologia 279/280:415-420.
Evers, D. C., J. D. Kaplan, M. W. Meyer, P. S. Reaman, W. E. Braselton, A. Major, N. Burgess, and A. M. Schuehammer. 1998. A geographic trend in mercury measured in Common Loon feathers and blood. Environmental Toxicology and Chemistry 17(2):173-183.
Facemire, C. F., T. S. Gross, and L. J. Guilette. 1995. Reproductive impairment in the Florida panther. Environmental Health Perspectives Supplement 4:79-86.
Gese, E. M., R. L. Ruff, and R. L. Crabtree. 1996. Foraging ecology of coyotes (Canis latrans): the influence of extrinsic factors and a dominance hierarchy. Canadian Journal of Zoology 74:769-783.
Hair, J. F., R. E. Anderson, and R. L. Tatham. 1987. Multivariate data analysis. Second edition. Macmillan, New York, New York, USA.
Heinz, G. H. 1976. Methylmercury: second generation reproductive and behavioral effects on mallard ducks. Journal of Wildlife Management 40(4):710-715.
_______ . 1979. Methylmercury: reproductive and behavioral effects on three generations of mallard ducks. Journal of Wildlife Management 43(2):394-401.
_______ . 1987. Mercury accumulation in mallards fed methylmercury with or without added DDE. Environmental Research 42:372-376.
Hontela, A., P. Dumont, D. Duclos, and R. Fortin. 1995. Endocrine and metabolic dysfunction in yellow perch, Perca flavescens, exposed to organic contaminants and heavy metals in the St. Lawrence River. Environmental Toxicology and Chemistry 14:725-731.
Kerekes, J. J. 1994. Fish-eating bird abundance in oligotrophic lakes in Kejimkujik National Park, Nova Scotia, Canada. Hydrobiologia 279/280:57-61.
Kerekes, J. J., and P. Schwinghammer. 1973. Aquatic resources inventory Kejimkujik National Park, Nova Scotia. Part 2. Lake drainage and morphometry. Canadian Wildlife Service, Environment Canada's Departmental Library, Hull, Québec, Canada.
Mager, J. N. 1995. A comparison of the time-activity budgets of breeding male and female Common Loons (Gavia immer). Thesis. Miami University, Oxford, Ohio, USA.
Martin, P., and P. Bateson. 1993. Measuring behaviour: an introductory guide. Second edition. Cambridge University Press, Cambridge, UK.
MathSoft. 1995. S-Plus for windows : advanced data analysis software. Version 3.3. MathSoft, StatSci Division, Seattle, Washington, USA.
McIntyre, J. W. 1975. Biology and behavior of the common loon (Gavia immer) with reference to its adaptability in a man-altered environment. Dissertation. University of Minnesota. Minneapolis, Minnesota, USA.
_______ . 1983. Nurseries: a consideration of habitat requirements during early chick-rearing period in Common Loons. Journal of Field Ornithology 54(3): 247-253.
McNicol, D. K., M. L. Mallory, and J. J. Kerekes. 1996. Canadian Wildlife Service LRTAP Biomonitoring Program, Part 3. Site locations, physical, chemical and biological characteristics. Technical Report Series Number 248, Canadian Wildlife Service, Environment Canada's Departmental Library, Hull, Québec, Canada.
Meyer, M. W., D. C. Evers, T. Daulton, and W. E. Braselton. 1995. Common Loons (Gavia immer) nesting on low pH lakes in Northern Wisconsin have elevated blood mercury content. Water, Air, and Soil Pollution 80:871-880.
Pfister, J. A., C. D. Cheney, and F. D. Provenza. 1992. Behavioral toxicology of livestock ingesting plant toxins. Journal of Range Management 45:30-36.
Scheuhammer, A. M., and P. J. Blancher. 1994. Potential risk to Common Loons (Gavia immer) from methylmercury exposure in acidified lakes. Hydrobiologia 279/280:445-455.
Scheuhammer, A. M., C. M. Atchison, A. H. K. Wong, and D. C. Evers. 1998. Mercury exposure in breeding Common Loons (Gavia immer) in central Ontario, Canada. Environmental Toxicology and Chemistry 17(2):191-196.
Sjølander, S., and G. Ågren. 1972. Reproductive behavior of the Common Loon. Wilson Bulletin 84(3):296-308.
Sokal, R. R., and F. J. Rohlf. 1995. Biometry : the principles and practice of statistics in biological research. Third edition. W.H. Freeman, New York, New York, USA.
Sutcliffe, S. A. 1978. Changes in status and factors affecting common loon populations in New Hampshire. Transactions of the Northeastern Section of the Wildlife Society 35:219-224.
Swain, E. B., D. R. Engstrom, M. E. Brigham, T. A. Henning, and P. L. Brezonik. 1992. Increasing rates of atmospheric mercury deposition in midcontinental North America. Science 257:784-787.
Tacha, T. C., P. A. Vohs, and G. C. Iverson. 1985. A comparison of interval and continuous sampling methods for behavioral observations. Journal of Field Ornithology 56: 258-264.
Tejning, S. 1967. Biological effects of methylmercury dicyandiamide-treated grain in the domestic fowl, Gallus gallus L. Oikos 8 (supplement):7-116.
U.S. EPA (Environmental Protection Agency). 1997. Mercury study report to Congress. Volume I - Executive Summary. Report Number EPA-452/R-97-003. Office of Air Quality Planning and Standards and Office of Research and Development, Washington, D.C., USA.
Vitousek, P. M., H. A. Mooney, J. Lubchenco, and J. M. Melillo. 1997. Human domination of Earth's ecosystems. Science 277:494-499.
Winchell, J. M., and T. H. Kunz. 1993. Sampling protocols for estimating time budgets of roosting bats. Canadian Journal of Zoology 71:2244-2249.
Address of Correspondent:
Joseph J. Nocera
Atlantic Cooperative Wildlife Ecology Research Network
Biology Department, Acadia University
Wolfville, Nova Scotia
B0P 1X0 Canada
Phone: (902)582-7997
Fax: (902)585-1059
023758n@acadiau.ca
*The copyright to this article passed from the Ecological Society of America to the Resilience Alliance on 1 January 2000.