|
|
|
Copyright © 1997 by The Resilience Alliance*
Andersen, A.N. 1997. Using Ants as bioindicators: Multiscale Issues in Ant Community Ecology. Conservation Ecology [online] 1(1): 8. Available from the Internet. URL: http://www.consecol.org/vol1/iss1/art8/
A version of this article in which text, figures, tables, and appendices are separate files may be found by following this link.
Insight Using Ants as bioindicators: Multiscale Issues in Ant Community Ecology Alan N. Andersen1
Cooperative Research Centre for the Sustainable Development of Tropical Savannas, Division of Wildlife and Ecology, CSIRO Tropical Ecosystems Research Centre
- Abstract
- Introduction
- Scale dependency in ant communities
- Implications for the use of ants as bioindicators
- Responses
- Literature Cited
KEY WORDS: ants; biodiversity surrogacy; bioindicators; community; diversity patterns; functional groups; multiscale; spatial scale.
There has been considerable recent interest among conservation biologists in the identification of robust indicators of the state of ecological systems that can be readily incorporated into land monitoring and assessment programs (Noss 1990, Spellerberg 1993, McKenzie et al. 1995). Attention has focused on the use of terrestrial invertebrates as bioindicators, because of their dominant biomass and diversity and their fundamental importance in ecosystem function (Disney 1986, Rosenberg et al. 1986, Majer 1989). Invertebrates have a long and successful history of use as biological indicators in aquatic systems (James and Evison 1979, Spellerberg 1993), but have only recently attracted similar interest on land (Kremen 1992, 1994, Williams 1993). A major exception has been the extensive use of ants as bioindicators, especially in Australia (Majer 1983, Greenslade and Greenslade 1984, Andersen 1990).
What exactly are bioindicators supposed to indicate? Traditionally, bioindicators have been used to assess ecosystem responses to environmental perturbation, often associated with human land use (Noss 1990, Spellerberg 1993, McKenzie et al. 1995). For 20 years, for example, the Australian mining industry has used ant species richness and composition as indicators of restoration success (Majer et al. 1984, Andersen 1997a), and these protocols have been exported to Brazil (Majer 1992) and South Africa (Majer and de Kock 1992). Patterns of ant species richness and composition at mine sites undergoing restoration have been shown to reflect recolonization by other invertebrate groups (Majer 1983, Andersen 1997a), as well as changes in soil microbial biomass (Andersen and Sparling 1997). More recently, forest management agencies in Australia have incorporated ants in monitoring programs associated with fire, grazing, and logging practices (Neumann 1992, York 1994, Vanderwoude et al. 1997). A recent feature of these studies is the use of functional groups, in relation to environmental stress and disturbance (Andersen 1995a), to assist in the prediction and interpretation of the responses of ant communities to land use (Andersen 1997a, Vanderwoude et al. 1997).
The term "bioindicator" also applies to the emerging discipline of biodiversity surrogacy, where potential "surrogate" or "target" taxa are examined for their capacity to provide an indication of total species diversity. The use of biodiversity surrogates has particular implications for conservation issues relating to reserve allocation and design, and ants have played a prominent role in these analyses (Andersen 1995b 1996, Abensperg-Traun et al.1996, Oliver and Beattie1996, Tennant de Alonso, in press).
Ant community ecology has a tradition of detailed studies in small plots, and only recently has attention shifted to patterns and processes operating at larger spatial scales (Andersen 1995a, 1997b, in press). The small-scale paradigm has produced much detailed information on the dynamics of particular communities, but, in my view, has provided poor research support for the use of ants as bioindicators. Such use requires a predictive understanding of ant community dynamics across a range of spatial scales, more than a detailed understanding of particular communities. My view should not be construed as blanket criticism of small-scale studies, which were never intended to be framed in a conservation context. Rather, it is a statement of the limited applicability of the traditional research paradigm to conservation ecology, at least for ants.
Here, I discuss how scale affects our understanding of ant communities and, consequently, their use as bioindicators. I consider spatial issues only, but issues relating to temporal variability are also important (Samways 1990).
Functional groups Community ecologists often classify species into functional groups that transcend taxonomic boundaries, thereby reducing the apparent complexity of ecological systems and allowing comparisons between communities with little species overlap. In a bioindicator context, functional groups can provide a widespread, predictive understanding of community responses to disturbance (Andersen 1997a). In animal communities, functional groups are typically "guilds," sets of species exploiting a common pool of resources (Terborgh and Robinson 1986), usually trophically based. Most ant species have similar foraging requirements; thus, trophically based guilds are of limited use in ant community studies (Andersen 1991a). Functional group classifications of plants, however, are based on a broad range of ecological characters, including life-form, morphology, reproductive behavior, and colonization ability, and are more appropriate models for functional group classifications of ants (Andersen 1991a, 1995a).
Functional group classifications designed to help interpret the dynamics of particular communities cannot necessarily be extrapolated to larger spatial scales. For example, ant ecologists often highlight the importance of competition to community structure, and focus their attention on the role of dominant species in communities (Fellers 1987, Savolainen and Vepsäläinen 1988; Andersen and Patel 1994). On a local scale, whichever species that is abundant and tends to win competitive interactions with other species, is considered dominant. As a result, a diverse array of taxa has been described as dominant (Hölldobler and Wilson 1990). On a global scale, however, dominant species are highly competitive taxa having their maximum expression under conditions of low environmental stress (factors limiting productivity) and disturbance (factors removing biomass; Grime 1979). For ants, such conditions are represented by hot and open environments experiencing low to moderate levels of disturbance, and the behaviorally dominant taxa that reach their maximum abundance at such sites are exclusively members of the sub-family Dolichoderinae (Andersen 1995a, 1997b, in press)
In other words, taxa that are behaviorally dominant at a local scale are often not at a global scale. Mound-building species of Formica are a good example. They are behaviorally dominant ants throughout the Holarctic, where they would correctly be described as dominant taxa in studies of local ant faunas (Rosengren and Pamilo 1983, Savolainen and Vepsäläinen 1988, Whittaker 1991). However, their dominance is restricted to cool-temperate regions, and they are poorly represented in arid and tropical habitats. At a global scale, species of Formica can therefore be considered as cold-climate specialists that have achieved behavioral dominance in relatively unfavorable habitats, in the absence of globally dominant dolichoderines (Andersen 1997b). Here, it is important to recognize that global dominance, where "global" defines the spatial scale at which such dominance operates, does not mean universal dominance, which implies local dominance everywhere.
Thus, functional group models of community structure are potentially dependent on scale. For functional groups, there is also a trade-off between generality, on one hand, and precision on the other, as is inevitable for ecological models dealing with multiscale systems (Holling 1964). Just as functional groups derived from detailed local studies (i.e., high local precision but limited generality) cannot necessarily be extrapolated to a global scale, functional groups from a global perspective (i.e., high generality but limited precision) may not be particularly useful for detailed studies of local communities. The concept of dolichoderines as globally dominant taxa, for example, does not translate meaningfully to studies of local Holarctic faunas, where dolichoderines are largely absent! Moreover, taxa belonging to different functional groups, from a global perspective, can behave similarly when considered locally. For example, highly competitive, mass-recruiting ants of the genus Solenopsis (subgenus Solenopsis) are climate specialists from a global perspective, because of their restricted biogeographic distribution (Andersen 1997b). Locally, however, their ecological behavior is similar to that of species of Monomorium, Pheidole, and Crematogaster, which are classified globally as Generalized Myrmicinae (Bestelmeyer and Wiens 1996).
Regulation of diversity In communities of sessile organisms such as plants (Loucks 1970, Grime 1973, Connell 1978, Tilman 1982) and colonial intertidal invertebrates (Paine 1974, Connell 1975, Huston 1979), local species diversity often exhibits a humped pattern in relation to gradients of stress and disturbance. It initially increases with reduced stress or disturbance, but then decreases as conditions allow highly competitive species to become so dominant that they exclude other species (see Abrams 1995 for a recent review). Such humped diversity patterns, mediated by competitive exclusion, have not been unequivocally documented in communities of mobile land animals, and, in most cases, are unlikely to occur (Fuentes and Jaksic 1988). Ants, however, appear to be exceptions, possibly because they share many characteristics with plants and other sessile taxa. They are modular organisms occupying fixed positions, co-occurring species have similar resource requirements, and competition is a prominent factor in community dynamics (Andersen 1991a). Humped diversity patterns in local ant communities have been documented along gradients of both stress (Fox et al. 1988, Andersen 1992) and disturbance (Majer 1985, Gallé 1991), with a reduction in diversity linked to exclusion by dominant species (see also Fox and Fox 1982).
There are two important scaling issues relating to humped patterns of diversity. First, the expression of a humped pattern requires a full range of productivity or disturbance (Rosenzweig and Abramsky 1993). If only a part of the range is sampled, then a variety of responses is possible, simply as artifacts of inadequate sampling (Fig. 1 ) increasing (only low levels of productivity sampled), no relationship (moderate levels only), or decreasing (high levels only)). The humped diversity model, therefore, cannot really be "tested" without a comprehensive coverage of stress or disturbance. Such a coverage may, in fact, be impossible to achieve, or even to define! Therefore, when testing for humped diversity patterns, it is more appropriate to ask specifically "Is the humped diversity model expressed over the spatial and temporal scales under investigation?" rather than more generally "Does the humped diversity model apply ?" For example, humped patterns of ant diversity might not be expressed in cold climates, as conditions might never be favourable enough for behaviorally dominant ants to exclude other species from local communities (Andersen 1995a, 1997b). Under such conditions, there might always be linear relationships between diversity on the one hand, and gradients of productivity and disturbance on the other. However, such a lack of expression of humped diversity patterns does not call into question the general validity of the model for ant communities.
FIG. 1. Hypothetical community where there is a humped relationship (dashed line) between species diversity and a gradient of productivity. If sampling is inadequate, there may, in fact, appear to be a positive relationship (a), no relationship (b), or a negative relationship (c), depending on the position along the productivity gradient.
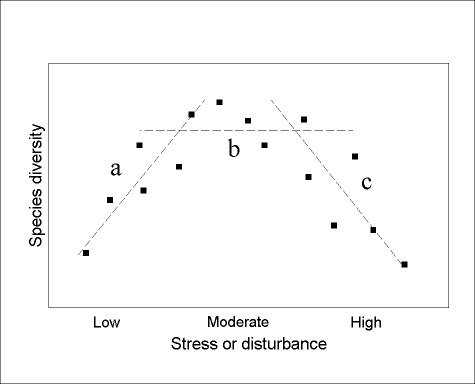
Second, humped diversity patterns apply to clearly circumscribed assemblages of species,
usually from topographically uniform environments located within a single bioclimatic
zone, whose local expression is determined primarily by variation in productivity and
disturbance. Such patterns cannot necessarily be expected to emerge from broader scale
analyses, where local processes are often overwhelmed by regional factors such as variation
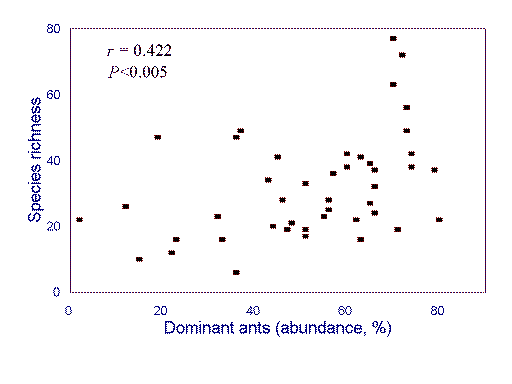
in climate and topography. For example, although humped patterns of richness appear
to be common in local Australian ant faunas, a continent-wide analysis reveals a (positive)
linear relationship between the abundance of behaviorally dominant species and local species
richness (Fig. 2
). This is explained by both local ant species richness and the abundance
of behaviorally dominant ants responding positively to increasing environmental
favorability, with regional processes overriding any local effects of competitive exclusion
(Andersen 1995a; see Ricklefs 1987, Holt 1993).
FIG. 2. Relationship between the abundance of behaviorally dominant ants and local
species richness in Australian ant communities (r 2 = 0.178, P <0.005). Redrawn from Andersen (1995a).
Measuring species richness and composition The standard procedure for describing site species richness and composition involves detailed measurements from smaller plots, which are deemed to be "representative" of the site as a whole. Unfortunately, exactly what constitutes a "site" is rarely made explicit, and it is seldom clear how many and what sized plots are required to be truly "representative," or indeed what specifically is to be "represented" (e.g., homogeneous plot vs. local heterogeneity). In most cases, the scaling functions required for reliable extrapolation from smaller scales to larger scales, including from homogeneity to heterogeneity, are unknown (Wiens 1989a, Rastetter et al. 1992).
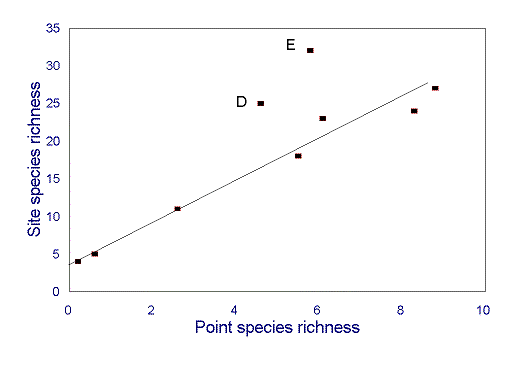
For ants, assessments of local species richness appear to be strongly scale dependent. For example, for most hectare-scale sites along an environmental gradient in southeastern Arizona, there is a very strong correlation between point (square-meter scale) and site species richness (Fig. 3 ). However, two sites do not conform to this relationship: they have very high site species richness, but only moderate point species richness (Fig. 3 ). Rates of species turnover between points are thus higher at these sites than at the others. Similar ant diversity patterns occur along gradients of disturbance in South American chaco (J.A. Wiens, personal communication).
FIG. 3. Relationship between point (m 2-scale) species richness (mean number of species in individual pitfall traps) and site (hectare scale) species richness for sites along an environmental gradient in southeastern Arizona. Sites D and E do not conform to the strong correlation evident at other sites. Data are results from 15 pitfall traps at each site, pooled across two 48-h sampling periods (from Andersen 1997b).
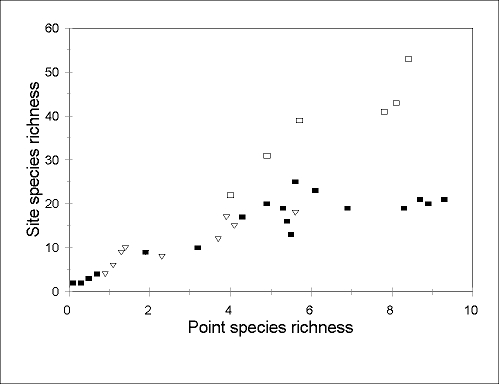
Rates of species turnover between points can also show substantial regional differences.
Point species richness at Arizonan sites appears generally similar to that at comparable
sites in Australia. Indeed, in the Arizonan study, lower diversity occurred at relatively cool
and wet, higher altitude sites (Andersen 1997b), where the relationship between
point and site species richness appears identical to that in cool-temperate Australia
(Fig. 4
). However, at richer (arid and seasonally arid) sites, site species richness is far
higher in Australia (Fig. 4
). In other words, the exceptional local species richness of
Australian arid-zone faunas appears to be due more to exceptional rates of species turnover
at very small scales than to exceptional point species richness. Rates of species turnover
can similarly vary at larger spatial scales. For example, the total number of granivorous
ant species in Australian deserts is an order of magnitiude greater than in North America,
despite comparable site species richness (Morton and Davidson 1988).
FIG. 4. Relationship between point species richness (as in Fig. 3) and site species richness in Arizona (closed squares; data from Andersen 1997b), compared with cool-temperate (open trinagles; data from Andersen 1986) and seasonal tropical (open squares; data from Andersen 1991b) sites in Australia. Data are from single sampling periods only, and sampling intensity is comparable in each case. The Australian cool-temperate and tropical data are results from 15 traps operated for 10 days, and 20 traps operated for 48 h, respectively.
Similar quantitative data are not available for larger spatial scales, but there appears to be
considerable variation in the proportional representation of local or regional faunas inside
small plots. One extreme seems to occur in the higher rainfall savannas of the Kakadu
region of Australia's Northern Territory. The regional savanna fauna is not exceptionally
rich (by Australian standards), but a large proportion of the fauna can be concentrated
inside very small plots. For example, up to 100 species have been recorded from individual
21 x 24 m (0.05-ha) plots (Andersen 1992), but other plots in the region tend to contain
nested subsets of this assemblage, rather than many additional species (A.N. Andersen,
unpublished data).
Therefore, rankings of sites according to species richness can be highly scale dependent.
Similar scaling issues also apply to species relative abundances. Unfortunately, however, I
know of no ant studies in which the sensitivity of measurements of relative abundances of
species to variation in plot size have been assessed. Such sensitivity is known for other
faunal groups, such as birds (Wiens 1989b).
Estimating species richness Due to the enormous logistical difficulties in obtaining measurements of total invertebrate biodiversity at any site, there has been considerable recent interest in identifying surrogates to provide estimates of total species richness. These estimates may be based on the number of higher level taxa (genera, families, etc.; Gaston and Williams 1993, Prance 1994, Williams and Gaston 1994), or on species richness within particular target taxa (Kremen 1994, Pearson 1994, Oliver and Beattie 1996).
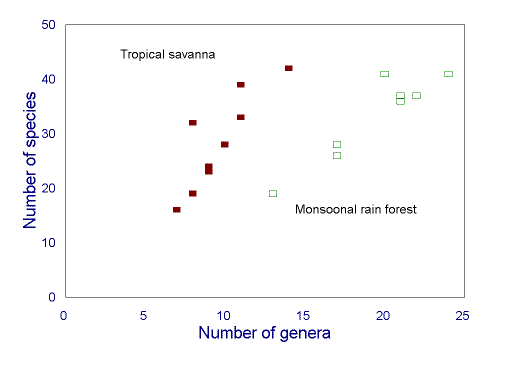
Surrogate measures of ant species richness are highly scale dependent. The relationship
between species richness and genus richness varies across a wide range of spatial scales. It
can be strong within regions, but varies substantially across regions (Fig. 5
), so that at a
continental scale, it has poor predictive power (Andersen 1995b). Within a region,
the relationship is especially strong among sites representing variation in disturbance of a
single habitat, but less so among sites encompassing variation between habitats (Andersen
1997c). There are particular ant genera whose site species richness is highly
correlated with the species richness of all ants, thus qualifying them as potential
target genera for estimates of total ant species richness. Unfortunately, however, the
reliability of these target genera can be scale specific (Table 1
). Camponotus, for
example, would appear to be a reliable target genus at a continental, but not regional, scale
in Australia, and the reverse is true for Rhytidoponera . As with genus richness,
the relationship between richness of target genera and total species richness is far stronger
among sites representing a single habitat type, but varying in disturbance, than among sites
encompassing variation between habitats (Andersen 1997c). Moreover, the relative
performance of target genera varies between these types of sites
(Table 2
).
TABLE 1: Correlations (r 2) between total numbers of ant species and numbers of species within particular target genera, for sites distributed throughout Australia (continental scale; data from Andersen 1995b , n = 10) compared with sites located within the Kakadu region of the Northern Territory of Australia (regional scale; data from Andersen 1997c , n = 22).
| Target genus | Continental | Regional |
|---|---|---|
| Camponotus | 0.83 | 0.07 |
| Iridomyrmex | 0.82 | 0.17 |
| Melophorus | 0.81 | 0.47 |
| Monomorium | 0.70 | 0.34 |
| Pheidole | 0.44 | 0.26 |
| Rhytidoponera | 0.07 | 0.38 |
TABLE 2. Ranking of target genera according to their ability to estimate total ant species richness at a site (hectare scale), based on correlations with within-genus richness (data from Andersen 1997c ). Two groups of regional sites are recognized, those representing a single habitat type but varying in disturbance, and those representing variation between habitats.
| Within Habitat | Between habitats |
|---|---|
| Melophorus | Melophorus |
| Rhytidoponera | Meranoplus |
| Monomorium | Rhytidoponera |
| Pheidole | Monomorium |
| Polyrhachis | Pheidole |
| Meranoplus | Iridomyrmex |
| Camponotus | Polyrhachis |
| Iridomyrmex | Camponotus |
FIG. 5. Relationship between ant genus and species richness in two biomes of the Australian seasonal tropics (redrawn from Andersen 1995b). The relationship is strong within each biome, but weak overall.
Using functional groups to assess ecological change The use of bioindicators to assess ecological change in relation to land use is most effective when supported by a predictive understanding of the organization of bioindicator communities. This allows the impact of anthropogenic disturbance to be distinguished from inherent site variability. More generally, it ensures correct interpretation of the "signal" provided by the bioindicator, especially given the limited replication available for many impact studies (Reynoldson et al. 1995, Wright 1995, Mac Nally 1996). The strong tradition of plot-scale research on ant communities has not provided such a predictive understanding.
Little or nothing is known of the species composition of most ant communities, let alone their dynamics. In most cases, predictive power is not possible at the species level, and will not be in the foreseeable future. Predictive power is possible, however, at the functional group level. Ant functional groups have been identified which vary predictably in relation to climate, soil, vegetation, and disturbance; these functional groups have formed the basis of continental and global analyses of community composition (Andersen 1995a, 1997b, in press). In addition to biogeographic comparisons, this broadscale predictive power, in relation to environmental stress and disturbance, has been usefully applied to plot-scale studies, such as the identification of taxa most likely to be limited by competitive interactions (Andersen 1992, Andersen and Patel 1994), and the responses of local communities to disturbance (e.g., Andersen and McKaige 1987, Andersen 1991b).
In a bioindicator context, the use of functional groups to provide broadscale predictive power is particularly valuable when the requirement of species-level precision is relatively low. The best examples concern mine site restoration (Andersen 1997a), where environmental disturbance has been extreme, and the goal of management is to produce self-sustaining ecosystems broadly similar to, but (given the relatively small area of land affected) not necessarily identical to, those occurring prior to disturbance. Ant functional groups show clear successional patterns in relation to time since rehabilitation (Andersen 1993), and the restoration of functional group composition might satisfy the "broadly similar" goal of restoration, even if species-level differences persist. Other examples include the monitoring of ecological responses to contrasting fire regimes, which produce markedly different profiles of ant functional groups (Andersen 1991b; Vanderwoude et al. 1997).
Global-scale functional groups can also be useful when greater sensitivity is required, but ants are more effective as bioindicators in these situations when functional groups are refined from more detailed analyses of ant community dynamics at regional or local scales. For example, the global functional group scheme has been modified to achieve more precision in a regional study of land use impacts in Argentinian chaco (Bestelmeyer and Wiens 1996). The modifications include subdividing some groups and, in the case of Solenopsis previously outlined, assigning taxa according to their regional, rather than global, role. Similarly, some predictable functional group patterns emerged from a study of local emission impacts from mining in South Australia, but a greater understanding of the ecology of key species was required for the most effective use of ants as bioindicators (Read 1996).
The requirement of species-level precision may also be low, and therefore the use of functional groups particularly valuable, when information on the ecological structure of bioindicator communities is more important than their specific composition. In ant communities, high levels of species turnover across sites often involve ecologically similar species, such that ecological structure is conserved. Such ecological structure might be a more reliable indicator than species composition. For example, changes in functional group composition of ants at disturbed sites in the Kakadu region of northern Australia sometimes provide a more reliable indication of the responses of other invertebrate groups than does ant species composition (Andersen 1997a).
Assessing species diversity Numerous scaling challenges confront the use of ant species richness as a general "biodiversity indicator" (c.f. Abensperg-Traun et al. 1996). At a continental scale, it seems to me absurd even to suggest that diversity patterns in any particular taxon might be representative of all others. Ants, for example, favor hot and open habitats; although ant species richness might reflect the richness of other arid-adapted taxa over very large spatial scales, it obviously would not for taxa preferring cool and moist habitats! Any general biodiversity indicator is therefore only likely to be reliable at regional or smaller scales, and this will be confounded by complex, nonlinear diversity patterns. For example, comparative "site" diversity for ants is highly scale dependent, with one "site" capable of being particulary rich at one scale, but not at another. Given such scale dependency of site rankings, what is the appropriate scale for comparison? A corollary is that any relationship between ant species richness and the richness of other groups is also likely to be scale dependent, as scaling functions are unlikely to be uniform across taxa. A similar argument applies to the use of surrogates (such as genus richness, or the species richness of target genera) to estimate ant species richness, where, again, diversity patterns are highly scale dependent. Whatever the case, the spatial scale at which biodiversity surrogacy is being examined must be clearly specified, and it cannot be assumed that the results will apply to other scales.
The conclusion of this analysis is that perceptions of fundamental patterns and processes in ant communities, and measurements of ant species richness, composition, and relative abundance, are all scale dependent, and that surrogates of total ant diversity are scale specific. The traditional small-plot paradigm of ecological research is unable to deal with these issues. As with conservation biology in general, the use of ants as bioindicators is best served by studies providing predictive power over a range of spatial scales, and this requires the integration of results from plot-scale research with the broader scale paradigms of biogeography, systematics, and evolutionary biology (Levin 1992, Brown 1995, Wiens 1997).
Responses to this article are invited. If accepted for publication, your response will be hyperlinked to the article. To submit a comment, follow this link. To read comments already accepted, follow this link.
Acknowledgments
I am grateful to Buzz Holling for encouraging me to write this and to John Wiens for many stimulating discussions on spatial scaling. I also thank John Wiens and three anonymous referees for their valuable comments on an earlier draft manuscript. This is publication no. 972 of the CSIRO Tropical Ecosystems Research Centre.
- Abensperg-Traun, M., G.W. Arnold, D.E. Steven, G.T. Smith, L. Atkins, J.J. Viveen, and M. Gutter. 1996. Biodiversity indicators in semiarid, agricultural Western Australia. Pacific Conservation Biology 2: 375-389.
- Abrams, P.A. 1995. Monotonic or unimodal diversity-productivity gradients: what does competition theory predict? Ecology 76 : 2019-2127.
- Andersen, A.N. 1986. Patterns of ant community organization in mesic southeastern Australia. Australian Journal of Ecology 11: 87-99.
- ______ . 1990. The use of ant communities to evaluate change in Australian terrestrial ecosystems: a review and a recipe. Proceedings of the Ecological Society of Australia 16: 347-357.
- ______ . 1991a . Parallels between ants and plants: implications for community ecology. Pages 539-558 in C.R. Huxley and D.C. Cutler, editors. Ant-plant interactions. Oxford University Press, Oxford, England.
- ______ . 1991b . Responses of ground-foraging ant communities to three experimental fire regimes in a savanna forest of tropical Australia. Biotropica 23: 575-585.
- ______ . 1992. Regulation of "momentary" diversity by dominant species in exceptionally rich ant communities of the Australian seasonal tropics. American Naturalist 140: 401-420.
- ______ . 1993. Ants as indicators of restoration success at a uranium mine in tropical Australia. Restoration Ecology 1: 156-167.
- ______ . 1995a . A classification of Australian ant communities, based on functional groups which parallel plant life-forms in relation to stress and disturbance. Journal of Biogeography 22: 2297-2311.
- ______ . 1995b. Measuring more of biodiversity: genus richness as a surrogate of species richness in Australian ant faunas. Biological Conservation 73: 39-43.
- ______ . 1997a. Ants as indicators of ecosystem restoration following mining: a functional group approach. Pacific Conservation Biology, in press.
- ______ . 1997b . Functional groups and patterns of organization in North American ant communities: a comparison with Australia. Journal of Biogeography, in press.
- ______ . 1997c . Measuring invertebrate biodiversity: surrogates of ant species richness in the Australian seasonal tropics. Memoirs of the Museum of Victoria 56: 355-359.
- ______ .In press. A global ecology of rain forest ants: functional groups in relation to stress and disturbance. Pages 000 in D. Agosti, J.D. Majer, L.E. Tennant de Alonso, and E. Schultz, editors. Measuring and monitoring biological diversity: standard methods for ants. Smithsonian Institution Press, Washington, D.C., USA.
- Andersen, A.N., R.W. Braithwaite, G.D. Cook, L.C. Corbett, R.J. Williams, M. Douglas, A.M. Gill, S. Setterfield, and W.J. Muller. In press. Fire research for conservation management in tropical savannas: introducing the Kapalga fire experiment. Australian Journal of Ecology.
- Andersen, A.N., and M.E. McKaige. 1987. Ant communities at Rotamah Island, Victoria, with particular reference to disturbance and Rhytidoponera tasmaniensis . Proceedings of the Royal Society of Victoria 99: 141-146.
- Andersen, A.N., and A.D. Patel. 1994. Meat ants as dominant members of Australian ant communities: an experimental test of their influence on the foraging success and forager abundance of other species. Oecologia 98: 15-24.
- Andersen, A.N., and G.P. Sparling. 1997. Ants as indicators of restoration success: relationship with soil microbial biomass in the Australian seasonal tropics. Restoration Ecology 5: in press.
- Bestelmeyer, B.T., and J.A. Wiens. 1996. The effects of land use on the structure of ground-foraging ant communities in the Argentine chaco. Ecological Applications 6: 1225-1240.
- Brown, J.H. 1995. Macroecology. University of Chicago Press, Chicago, Illinois, USA.
- Connell, J.H. 1975. Some mechanisms producing structure in natural communities. Pages 460-490 in M.L. Cody and J.M. Diamond, editors.Ecology and evolution of communities. Belknap, Cambridge, Massachusetts, USA.
- ______ . 1978. Diversity in tropical rain forests and coral reefs. Science 199: 1302-1310.
- Disney, R.H.L. 1986. Assessments using invertebrates: posing the problem. Pages 271-293 in M.B. Usher, editor. Wildlife conservation evaluation. Chapman and Hall, London, England.
- Fellers, J.H. 1987. Interference and exploitation in a guild of woodland ants. Ecology 68: 1466-1478.
- Fox, B.J., E. Archer, and M.D. Fox. 1988. Ant communities along a moisture gradient. Pages 661-667 in F. di Castri, C. Floret, S. Rambal, and J. Roy, editors. Time scales and water stress. International Union of Biological Sciences, Paris, France.
- Fox, B.J., and M.D. Fox. 1982. Evidence for interspecific competition influencing ant species diversity in a regenerating heathland. Pages 99-110 in R.C. Buckley, editor. Ant-plant interactions in Australia. Dr W. Junk Press, The Hague, The Netherlands.
- Fuentes, E.R., and F.M. Jaksic. 1988. The hump-backed species diversity curve: why has it not been found among land animals? Oikos 53: 139-143.
- Gallé, L. 1991. Structure and succession of ant assemblages in a north European sand dune area. Holarctic Ecology 14 : 31-37.
- Gaston, K.J., and P.H. Williams. 1993. Mapping the world's biodiversity: the higher taxon approach. Biodiversity Letters 1: 2-8.
- Greenslade, P.J.M., and P. Greenslade. 1984. Invertebrates and environmental assessment. Environment and Planning 3: 13-15.
- Grime, J.P.1973. Competitive exclusion in herbaceous vegetation. Nature 242: 344-347.
- ______ . 1979. Plant strategies and vegetation processes. Wiley, Chichester, England.
- Hölldobler, B., and E.O. Wilson. 1990. The ants. Belknap, Cambridge, Massachusetts, USA.
- Holling, C.S. 1964. The analysis of complex population processes. Canadian Entomologist 96: 335-347.
- Holt, R.D. 1993. Ecology at the mesoscale: the influence of regional processes on local communities. Pages 77-88 in R.E. Ricklefs and D. Schluter, editors. Species diversity in ecological communities: historical and geographical perspectives. University of Chicago Press, Chicago, Illinois, USA.
- Huston, M. 1979. A general hypothesis of species diversity. American Naturalist 113: 81-101.
- James, A., and L. Evison, editors. 1979. Biological indicators of water quality. John Wiley, Chichester, England.
- Kremen, C. 1992. Assessing the indicator properties of species assemblages for natural areas monitoring. Ecological Applications 2: 203-217.
- ______ . 1994. Biological inventory using target taxa: a case study of the butterflies of Madagascar. Ecological Applications 4: 407-422.
- Levin, S.A. 1992. The problem of pattern and scale in ecology. Ecology 73: 1943-1967.
- Loucks, O.L. 1970. Evolution of diversity, efficiency and community stability. American Zoologist 10: 17-25.
- Mac Nally, R. 1996. A winter's tale: among-year variation in bird community structure in a southeastern Australian forest. Australian Journal of Ecology 21 : 280-291.
- Majer, J.D. 1983. Ants: bioindicators of mine site rehabilitation, land use and land conservation. Environmental Management 7: 375-383.
- ______ .1985. Recolonization by ants of rehabilitated mineral sand mines on North Stradbroke Island, Queensland, with particular reference to seed removal. Australian Journal of Ecology 10: 31-48.
- ______ ., editor. 1989. Animals in primary succession: the role of fauna in reclaimed lands. Cambridge University Press, Cambridge, England.
- ______ . 1992. Ant recolonization of rehabilitated bauxite mines of Poços de Caldas, Brazil. Journal of Tropical Ecology 8: 97-108.
- Majer, J.D., J.E. Day, E.D. Kabay, and W.S. Perriman. 1984. Recolonization by ants in bauxite mines rehabilitated by a number of different methods. Journal of Applied Ecology 21: 355-375.
- Majer, J.D., and A.E. de Kock. 1992. Ant recolonization of sand mines near Richards Bay, South Africa: an evaluation of progress with rehabilitation. South African Journal of Science 88: 31-36.
- McKenzie, D.H., D.E. Hyatt, and V.J. McDonald, editors.1995. Ecological indicators. Chapman and Hall, London, England.
- Morton, S.R., and D.W. Davidson. 1988. Comparative structure of harvester ant communities in arid Australia and North America. Ecological Monographs 58 : 19-38.
- Oliver, I., and A.J. Beattie. 1996. Designing a cost-effective invertebrate survey: a test of some methods for the rapid assessment of invertebrate biodiversity. Ecological Applications 6: 594-607.
- Neumann, F.G.1992. Responses of foraging ant populations to high intensity wildfire, salvage logging, and natural regeneration processes in Eucalyptus regnans regrowth forest of the Victorian central highlands. Australian Forestry 55: 29-38.
- Noss, R.N. 1990. Indicators for monitoring biodiversity: a hierarchical approach. Conservation Biology 4: 355-364.
- Paine, R.T. 1974. Intertidal community structure: experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia 15: 93-120.
- Pearson, D.L.1994. Selecting indicator taxa for the quantitative assessment of biodiversity. Philosophical Transactions of the Royal Society of London, Series B 345: 75-79.
- Prance, G.T. 1994. A comparison of the efficacy of higher taxa and species numbers in the assessment of biodiversity in the neotropics. Philosophical Transactions of the Royal Society of London, Series B 345: 89-99.
- Rastetter, E.B, A.W. King, B.J. Cosby, G.M. Hornberger, R.V. O'Neill, and J.E. Hobbie. 1992. Aggregating fine-scale ecological knowledge to model coarser scale attributes of ecosystems. Ecological Applications 2: 55-70.
- Read, J.J. 1996. Use of ants to monitor environmental impacts of salt spray from a mine in arid Australia. Biodiversity and Conservation 5: 1533-1543.
- Reynoldson T.B., R.C. Bailey, K.E. Day, and R.H. Norris. 1995. Biological guidelines for freshwater sediment based on BEnthic Assessment of S edimen T (the BEAST) using a multivariate approach for predicting biological state. Australian Journal of Ecology 20: 198-219.
- Ricklefs, R.E. 1987. Community diversity: relative roles of local and regional processes. Science 235: 167-171.
- Rosenberg, D.M., H.V. Danks, and D.M. Lehmkuhl.1986. Importance of insects in environmental impact assessment. Environmental Management 10: 773-783.
- Rosengren, R., and P. Pamilo. 1983. The evolution of polygyny and polydomy in mound-building Formica ants. Acta Entomologica Fennica 42: 65-77.
- Rosenzweig, M.L., and Z. Abramsky. 1993. How are diversity and productivity related? Pages 52-65 in R.E. Ricklefs and D. Schluter, editors. Species diversity in ecological communities: historical and geographical perspectives. University of Chicago Press, Chicago, Illinois, USA.
- Samways, M.J. 1990. Species temporal variability: epigaeic ant assemblages and management for abundance and scarcity. Oecologia 84: 482-490.
- Savolainen, R., and K. Vepsäläinen. 1988. A competition hierarchy among boreal ants: impact on resource partitioning and community structure. Oikos 51: 135-155.
- Schneider, D.C. 1994. Quantitative ecology: spatial and temporal scaling. Academic Press, San Diego, California, USA.
- Spellerberg, I.F. 1993. Monitoring ecological change. Cambridge University Press, Camridge, England.
- Terbourgh, J., and S. Robinson. 1986. Guilds and their utility in ecology. Pages 65-90 in J. Kikkawa and D.J. Anderson, editors. Community ecology: patterns and processes. Blackwell Scientific Publications, Melbourne, Australia.
- Tennant de Alonso, L.E. In press. Ants as biodiversity indicators. Pages 000 -000 in D. Agosti, J.D. Majer, L.E. Tennant de Alonso, and E. Schultz, editors. Measuring and monitoring biological diversity: standard methods for ants. Smithsonian Institution Press, Washington, D.C., USA.
- Tilman, D.1982. Resource competition and community structure. Princeton University Press, Princeton, New Jersey, USA.
- Vanderwoude, C., A.N. Andersen, and A.P.N. House. 1997. Ant communities as bioindicators in relation to fire management of spotted gum ( Eucalyptus maculata Hook.) forests in southeast Queensland. Memoirs of the Museum of Victoria 56: 671-675.
- Whittaker, J.B. 1991. Effects of ants on temperate woodland trees. Pages 67-79 in C.R. Huxley and D.F. Cutler, editors. Ant-plant interactions. Oxford University Press, Oxford, England.
- Wiens, J.A. 1989a . Spatial scaling in ecology. Functional Ecology 3: 385-397.
- ______ . 1989b . The ecology of bird communities. Cambridge University Press, Cambridge, England.
- ______ .. 1997. The emerging role of patchiness in conservation biology. Pages 93-107 in S.T.A. Pickett, R.S. Ostfeld, M. Shachak, and G.E. Likens, editors. Enhancing the ecological basis for conservation: heterogeneity, ecosystem function, and biodiversity. Chapman and Hall, New York, New York, USA.
- Williams, K.S. 1993. Use of terrestrial arthropods to evaluate restored riparian woodlands. Restoration Ecology 1: 107-116.
- Williams, P.H., and K.J. Gaston. 1994. Measuring more of biodiversity: can higher taxon richness predict wholesale species richness? Biological Conservation 67: 211-217.
- Wright, J.F. 1995. Development and use of a system for predicting the macroinvertebrate fauna in flowing waters. Australian Journal of Ecology 20: 181-197.
- York, A. 1994. The long-term effects of fire on forest ant communities: management implications for the conservation of biodiversity. Memoirs of the Queensland Museum 36: 231-239.
- Abrams, P.A. 1995. Monotonic or unimodal diversity-productivity gradients: what does competition theory predict? Ecology 76 : 2019-2127.
Address of Correspondent:
Alan N. Andersen
Cooperative Research Centre for the Sustainable Development of Tropical Savannas
Division of Wildlife and Ecology
CSIRO Tropical Ecosystems Research Centre
PMB 44 Winnellie, NT 0821, Australia
voice: 618-89448431
fax: 618-89470052
alan.andersen@terc.csiro.au
*The copyright to this article passed from the Ecological Society of America to the Resilience Alliance on 1 January 2000.